

It may be necessary to shake the test tubes again to transfer more of the halogen from the water to the hydrocarbon layer.Īcidic and bleaching properties of halogen solutions Observe and record the colour of each layer. Add equal volumes of hydrocarbon solvent to each tube, stopper the tube and, holding your thumb over the bung, shake the mixture by inverting the test tube a few times.
At the end of the experiments all mixtures and solutions should be returned to a suitable waste container in a fume cupboard for safe disposal.The halogen solutions can be diluted further to minimise the amount of chlorine or bromine fumes given off but should not be so dilute that their distinctive colours are not clearly visible in the test tubes (a white background may be needed for chlorine water).Each group of students should be supplied with stoppered test tubes containing about 10 cm 3 of each of the aqueous solutions of the halogens and one of cyclohexane (optional).Cyclohexane (HIGHLY FLAMMABLE, HARMFUL, DANGEROUS FOR THE ENVIRONMENT) or other suitable non-polar solvent, about 10 cm 3 (see note 1).Universal indicator paper (about 2 cm strips), x3.Half a test tube of 0.1 M solutions of each of the following:.About 10 cm 3 of each of the following halogen solutions in stoppered test tubes (see notes 1 and 2):.If it is done as a class experiment you should allow 30 minutes. If the activity is done as a demonstration it should take around 15 minutes. Investigating the solubility of the halogens in a non-polar solvent can be left out, or only shown as a demonstration. It can be done as a demonstration or as a class experiment.
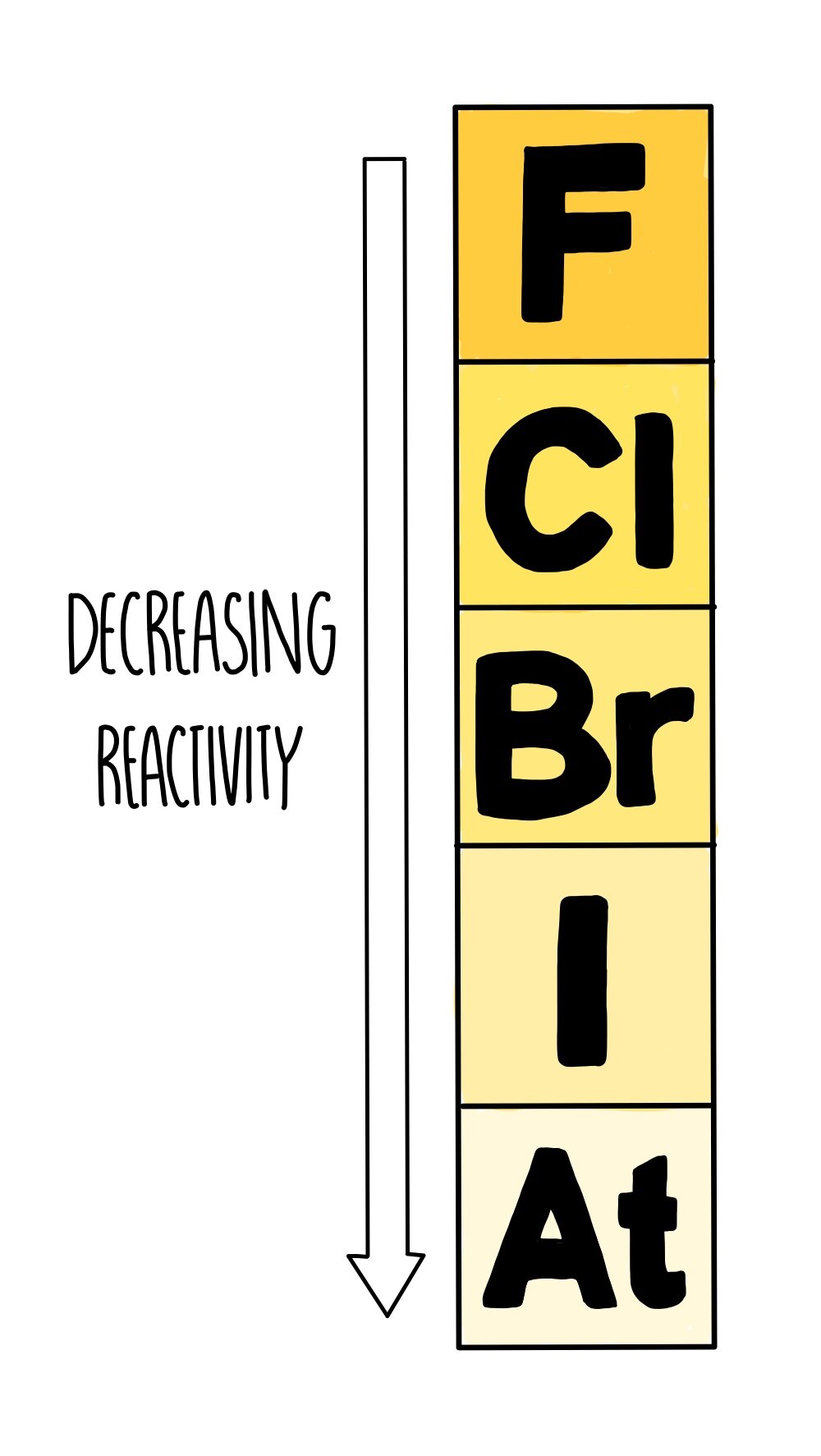
#Halogen reactivity trend snar series
This series of simple experiments illustrates some of the chemical properties of the halogens following an introduction to the physical properties of the Group 17 elements. These displacement reactions are used to establish an order of reactivity down Group 17 of the periodic table.

Halogens react to a small extent with water, forming acidic solutions with bleaching properties. They also undergo redox reactions with metal halides in solution, displacing less reactive halogens from their compounds. RSC Yusuf Hamied Inspirational Science Programme.Introductory maths for higher education.The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
